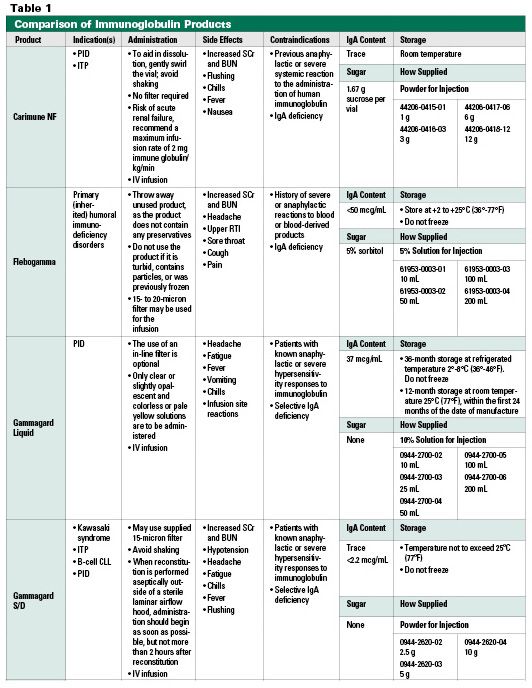
Here is a link with IVIG products and their. IVIG products may differ in their pharmaceutical properties osmolality pH sodium content stabilizer IgA content which can affect safety and tolerability.
13 When selecting an IVIG treatment individual patient characteristics should be considered.
. We summarize and assess the 23 case reports documented in the literature that have described anaphylactic reactions in immunodeficient patients receiving IVIG since 1962 until currently and make a comparison of their immunoglobulin levels IgG anti-IgA IgE anti-IgA concentration of IVIG IgA content in IVIG method used to detect antibodies. Subclass content IgG1 61 IgG2 36 IgG3 3 IgG4 1 IgG1 476-562 IgG2 415-495 IgG3 13-16. Known systemic hypersensitivity to human albumin in the PH20 solution.
13 IVIG treatments are distinct plasma products with patient tolerance differing from one brand to another. Between 1968 and 2006 40 cases of anti-IgA-related anaphylaxis to blood transfusions Ig replacement therapy and other plasma products have been summarized following library searches Three of these cases were attributed to IVIg treatment Reviewing the literature between 1987 and 2006 on CVID patients in whom severe IgAD is often associated. The final formulation can differ among IVIGs meaning they are not interchangeable.
This page is an in-depth guide about all the IVIG brands and their major differences. Contraindicated in patients with history of prior systemic allergic reaction to IVIG products or a history of IgA deficiency pH of 4-45 Additives. Privigen is contraindicated in IgA-deficient patients with antibodies to IgA Convenient ready-to-use 10 IVIg that can be stored at room.
You may use another IVIG product with lower IgA content. With IVIG administration every 3 or 4 weeks peak concentrations. IVIG are subjected to industrial manipulation processes of inactivation and chemical and physical removal of bacteria and viruses.
Watch A Video Discussing The Difference Between IVIg SCIg. Ad See The Dosing Flexibility Of A Trusted Immunoglobulin. IVIg Proven effective Privigen is the first and only IVIg designed with proline stabilization Privigen is approved for the treatment of PI CIDP and chronic ITP Low IgA content Convenient ready-to-use 10 IVIg that can be stored at room temperature Important Safety Information for Privigen Privigen is indicated for the treatment of.
Explore The Safety Profile Of A Trusted IVIg. Not all intravenous immune globulin IVIG formulations are interchangeable 1-3. November 2 2021.
Ad Learn How Self-Infusing This Ig Therapy Can Put Administration In Your Hands. IgA is not problematic for most people6 However in patients who are IgA deficient IgA can cause the formation of anti-IgA antibodies that can cause anaphylactoid reactions upon infusion of IVIG which would result from the. Some IVIG products are given intravenously.
Known hypersensitivity to hyaluronidase including PH20 of HYQVIA. IgA-deficient patients with antibodies to IgA and a history of hypersensitivity. Persons with isolated IgA deficiency.
Severe anaphylactic reactions are rare and have been reported when using IVIG products due to the presence of IgG or IgE anti-IgA antibodies in patients with IgA deficiency7980 Paradoxical use of IgA-depleted IVIG or a SCIG preparation is suggested in the treatment of. IVIg Used in US Hospitals Since 20101 Proven effective Privigen is the first and only IVIg designed with proline stabilization Privigen is approved for the treatment of PI CIDP and chronic ITP Low IgA content. However anaphylaxis to IVIG is exceedingly rare and in the majority of cases anti-IgA antibodies are likely not the culprit.
Precipitation and removal of fraction III of the cold ethanol process solventdetergent treatment 35 nm nanofiltration Liquid Refrigerate between 2 to 8C. It is estimated that one third of patients with IgA deficiency have detectable anti-IgA antibodies. The available IVIG and SCIG products differ in their pharmaceutical properties eg pH osmolality IgA content sodium content and stabilizer which can affect safety and tolerability in some patients.
Sodium Content Osmolarity Osmolality PH IgA Content Approved Method of Administration Bivigam ADMA Biologics Modified classical Cohn Method 6 Oncley Method 9 fractionation procedure. Severe thrombocytopenia or any coagulation disorder or. Although the majority of renal adverse events have occurred with sucrose containing IVIg products caution is advised during administration of any IVIg product.
The pharmacokinetics of Ig also differ based on the route of administration. IgA Content All IG products contain varying amounts of IgA one of the five classes of antibodies found in the blood. IVIG is the acronym for Intravenous Immune globulin and is mainly given in autoimmune or immunological disorders diseases in which your immune system attacks your own cells or organs mistakenly.
Ad Review The Clinical Studies Supporting The Use Of This IVIg For CIDP PI Chronic ITP. The available IVIG and SCIG products differ in their pharmaceutical properties eg pH osmolality IgA content sodium content and stabilizer which can affect safety and tolerability in some. I believe the IgA content is a factor in adverse reactions though.
In IgA deficient patients product with the lowest IgA level should be. Watch A Self-Infusion Video Learn About The Benefits Of Self-Infusing Ig.

Intravenous And Subcutaneous Immunoglobulin Treatment Options

Intravenous And Subcutaneous Immunoglobulin Treatment Options

Intravenous And Subcutaneous Immunoglobulin Treatment Options
Ivig The Complete Guide Ameripharma Specialty Care
Specialty Pharmaceuticals Immunoglobulin Therapy

Intravenous And Subcutaneous Immunoglobulin Treatment Options
0 comments
Post a Comment